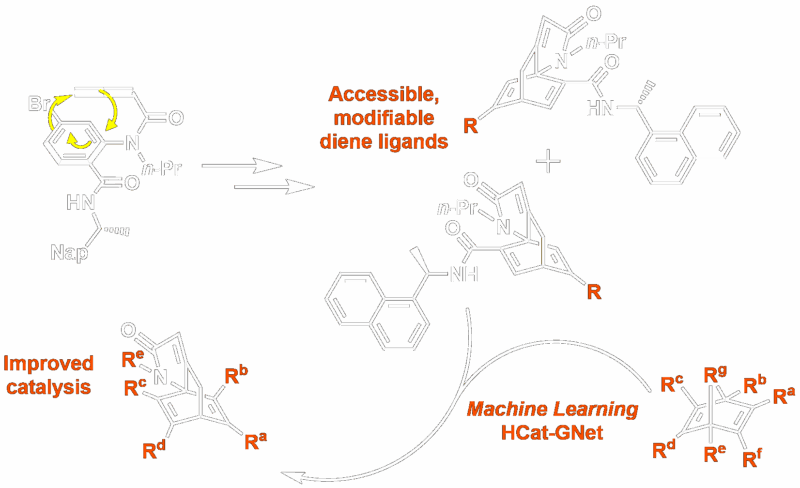
Scalable Chiral Ligands and AI-Explainable Chemical Space
As part of a new series highlighting the brilliant research that the chemistry and life sciences community undertake on a daily basis, in small part supported by Fluorochem materials and reagents: here we point the spotlight on work performed in the lab of Professor Simon Woodward, at the University of Nottingham, developing novel synthetic and computational approaches to improved asymmetric ligands for transition metal catalysis.
Creating molecules with well-defined stereocentres is of fundamental importance to synthetic chemistry. One way chemists achieve this is through asymmetric catalysis, often using transition metal catalysts, complexed with chiral ligands. Chiral dienes are an important class of ligands, often showing greater catalytic activity than alternatives, such as phosphines. However, lengthy syntheses and difficulties in isolating enantiomerically pure materials have limited their use.
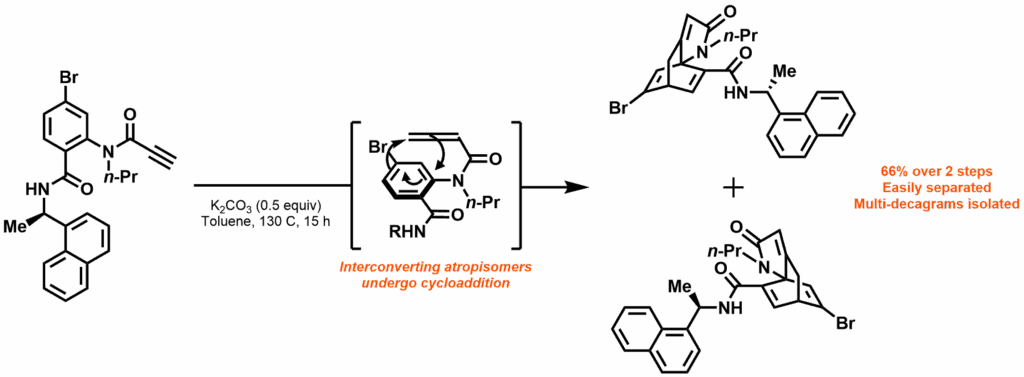
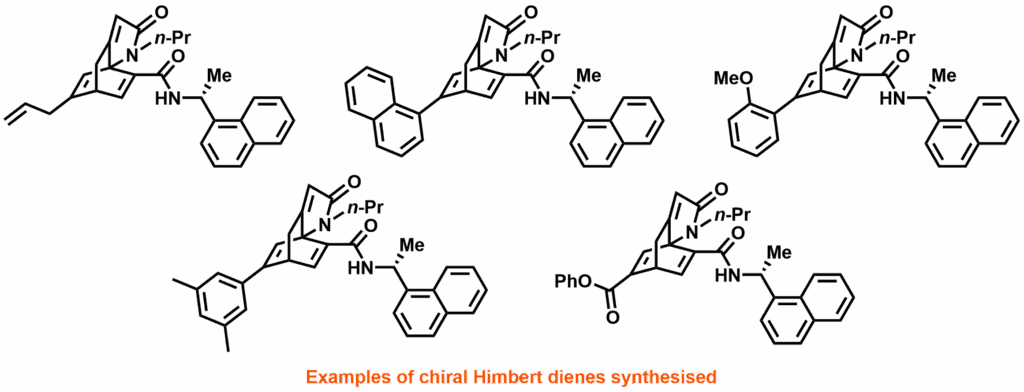
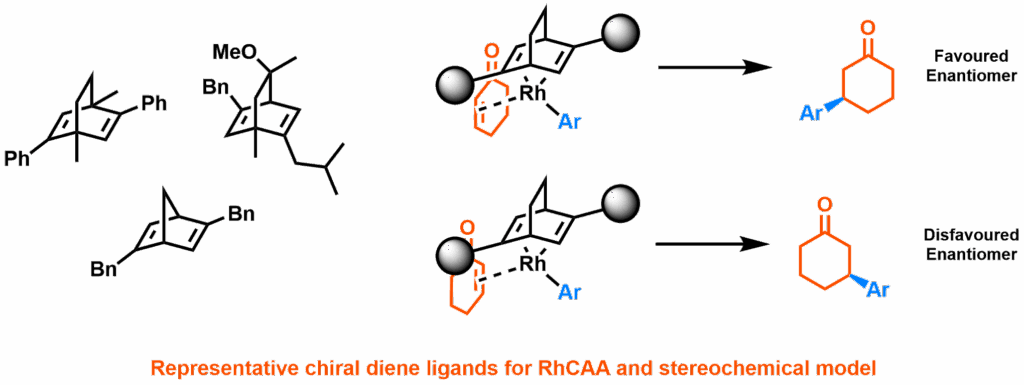
In this work by Rit et al, reported in Advanced Synthesis & Catalysis, the Himbert cycloaddition was recognised as a route to the core structure of many chiral diene ligands. The reaction dearomatises benzene through an intramolecular Diels-Alder reaction with a pendant allenecarboxanilide. By introducing a chiral auxiliary, this reaction was exploited to produce easily separable pseudoenantiomers in multi-decagram quantities. Using a bromobenzene starting material produces alkenyl bromide products; allowing these “Himbert diene” ligands to be further functionalised through palladium catalysis and consequently providing access to a wide range of novel diene ligands.
The dimeric rhodium(I) complexes formed from these ligands were used in the rhodium-catalysed asymmetric 1,4-addition (RhCAA) of aryl boronic acids to 2-cyclohexenone, with high yields and enantioselectivities – competitive with the best-performing diene complexes reported so far. Chemistry with more challenging electrophiles and alternative boronates was successful, including the allenylation of cyclic imines (a reaction not previously attempted with chiral diene ligands). These also produced similarly exceptional results, in some cases surpassing any previously recorded rhodium(I) or palladium(II) catalysed reactions.

In short, the simplicity and flexibility of synthesis of these new ligands, combined with the potent catalytic complexes that they produce, shows great promise and the potential to expand the synthetic chemist’s toolbox. The ease with which they can be modified means that they can be readily optimised for numerous, different catalytic processes.
Graph Neural Networks for Accelerated Catalyst Development
This ease of modification in a relatively undeveloped area of asymmetric catalysis provided the perfect opportunity to explore the use of machine learning to accelerate ligand design. Writing in iScience, Aguilar-Bejarano et al present a Homogeneous Catalyst Graph Neutral Network (HCat-GNet), just such a machine learning model for aiding ligand optimisation. Usually this process is extremely time-consuming, involving the cyclic process of hypothesising changes to activity through structural changes, synthesis, testing followed by revaluation of the ligand performance.
Quantitative structure-activity relationship (QSAR) studies enabled by machine learning are becoming increasingly popular in the chemical sciences, but their application to asymmetric catalysis remains limited. Previous methods have only dealt with restricted ligand changes or have required large amounts of empirical data on the reaction components. This has reduced the problems these models can be applied to, a problem further exacerbated by a lack of human-interpretable results.
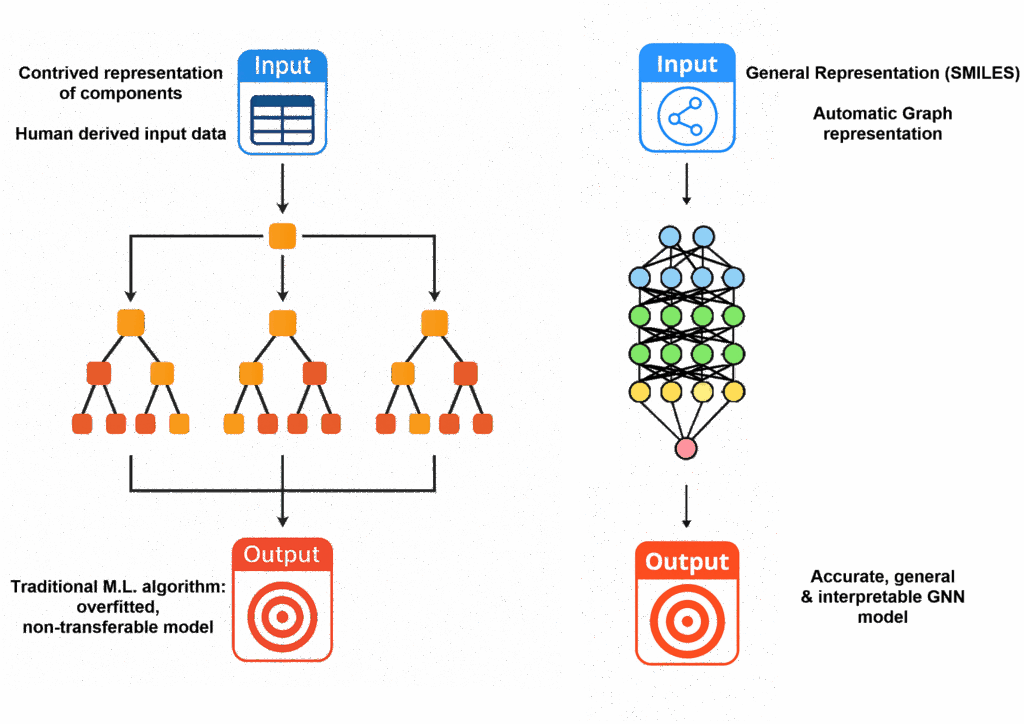
Graph Neural Networks have been shown to be effective at predicting QSARs for chemical reactions, producing accurate reaction yields and regioselectivities, but still often requiring computationally demanding density functional theory (DFT) calculations. This work shows how HCat-GNet can use only the SMILES of reaction components to predict the absolute stereochemistry, and selectivity (expressed in the chemist-friendly format of ΔΔG‡ values). The ‘Graphs’ that feed the Graph Neural Network contain information on all atoms contained within the ligand, substrate and nucleophile; the necessary information (connectivity, hybridisation etc) is all contained within the SMILES notation.
The new Himbert diene ligands create an ideal testing ground, being novel, but still applicable to the established reaction RhCAA. Along with plentiful historic data, it also provided an existing stereochemical model – allowing results to be compared with a human chemist’s intuition, as well as benchmarked against pre-existing machine learning approaches.
Taking a library of 668 historical reactions for initial training-testing, the GNN was confirmed to model these data, alongside existing machine learning approaches. Importantly, the novel Himbert Diene ligands were not included in the training, and it was shown that HCat-GNet was capable of accurately predicting enantioselectivities of these ‘unseen’ reactions. Interestingly, due to the bias in published literature for high enantioselectivities, predictions at lower selectivities were less accurate. Ultimately, it proved consistently capable of identifying the best ligand from a group of similar molecules, replicating the conclusions made by lab chemists in a fraction of the time.
Further insights were possible with the use of Explainable Artificial Intelligence: GNNExplainer was applied to recognise the factors (for example, ligand substituent position and substitutions) that led to higher enantioselectivity. Similarly, Shapley Value Sampling (SHAP) could assess the impact of changing the electronics of the diene ligands.
While focusing on RhCAA in this publication, the authors expand its use to further asymmetric reactions, featuring different ligands. The model maintained competitive accuracy against existing approaches with minor modifications. Therefore, the interesting feature of this approach, requiring only SMILES representation of the reacting molecules, is its generality. It can be expanded to optimise a range of metal-catalysed asymmetric reactions, even in situations where the mechanism is unknown. The authors hope that this accessible approach aids synthetic chemists in their approach to methodology and catalyst development.
Dr M. Tomsett – Technical Liason Officer
R. K. Rit, H. Li, S. P. Argent, K. M. Wheelhouse, S. Woodward, H. W. Lam, Adv. Synth. Catal. 2023, 365, 1629.
E. Aguilar-Bejarano, E. Özcan, R. K. Rit, H. Li, H. W. Lam, J. C. Moore, S. Woodward, G. Figueredo. iScience, Volume 28, Issue 3, 111881
Woodward Research Group
Eduardo Aguilar-Bejarano